Search

Search

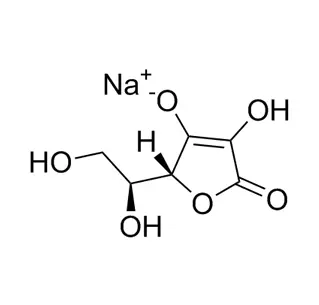
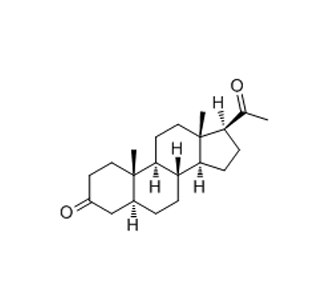
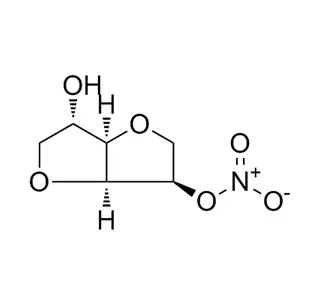
Pharmaceutical intermediate is a semi-finished product. In the pharmaceutical field, it refers to a material produced in the process steps of active pharmaceutical ingredients, but can not be used directly. It can become an active pharmaceutical ingredient only after further molecular change or refining. It is a reaction product, also can be a reaction raw material. The pharmaceutical intermediate can be separated or not. As can be seen from the definition, pharmaceutical intermediate is the key product of the previous process of making active pharmaceutical ingredients, which is different from the active pharmaceutical ingredients. Pharmacopoeia has the detection method of active pharmaceutical ingredients, but there is no detection method of pharmaceutical intermediate.

Active pharmaceutical ingredients refer to the raw ingredients used to produce various preparations. It is an active ingredient in preparations. It is used in drug manufacturing, which can be pure or mixture, and becomes an active ingredient of medicine when used in pharmacy. Active pharmaceutical ingredients like malaria treatment artemisinin have pharmacological activity or other direct effects in disease diagnosis, treatment, symptom relief, treatment or disease prevention, or can affect the function and structure of the body. Active pharmaceutical ingredients are prepared by chemical synthesis, plant extraction or biotechnology. Only when it is processed into pharmaceutical preparations can it become a medicine for clinical application.

Pharmaceutical intermediate: A material produced in the process of active pharmaceutical ingredients, which must undergo further molecular change or refining to become active pharmaceutical ingredients. Pharmaceutical intermediate can be separated or not separated.
Active pharmaceutical ingredients: Active medicinal ingredients (active pharmaceutical ingredients) refer to substance or mixture of substances intended to be used in medicine manufacturing, and becomes an active ingredient of medicine when used in pharmacy. This substance has pharmacological activity or other direct effects in disease diagnosis, treatment, symptom relief, treatment or disease prevention, or can affect the function and structure of the body.
It can be seen from the definition that pharmaceutical intermediate is the key product of the previous process of making active pharmaceutical ingredients, which is different from the structure of active pharmaceutical ingredients. In addition, the Pharmacopoeia has the detection method of active pharmaceutical ingredients, but there is no detection method of pharmaceutical intermediate. When it comes to certification, at present, FDA requires that pharmaceutical intermediate must be registered, while COS does not. However, the CTD document should have a detailed process description of the pharmaceutical intermediate. In China, there is no mandatory GMP requirement for pharmaceutical intermediate.

hydroxypropyl beta cyclodextrin

Quick Links
Add:
E-mail:
 English
English  Español
Español  français
français  العربية
العربية